Cleanroom Validation
Streamlined Validation Services for Pharmaceutical Cleanrooms & Controlled Environments
As pharmaceutical cleanroom validation specialists, we at PDVD Consultancy can help you ensure that your pharmaceutical facility is operating as efficiently as possible. Pharmaceutical Cleanrooms are crucial places for several sectors, including biotechnology, microelectronics, and pharmaceuticals, where preserving air quality is crucial to product integrity.
PDVD is established as one of the leading Cleanroom validation, Equipment qualification, Temperature mapping services provider company in india. We provide an extensive range of cleanroom validation, Equipment qualification, Temperature mapping services with all testing carried out to the highest standard by fully trained and competent engineers.
Our Cleanroom Validation & other validation / qualification methods precisely comply with Regulatory bodies like ISO 14644-1 and guideline agencies such as MHRA, FDA, EU GMP, WHO-GMP and Schedule M for all room classification and so many other Industry-Specific Standards. PDVD has expertise not only in Pharmaceutical cleanroom but also Modular Operation Theatre, Laboratory, and Various equipment like Bio-safety cabinet, Laminar air flow equipment, Fume cupboard etc., Our pharmaceutical validation services allow you to rest easy knowing that your cleanroom conforms to industry and legal standards.
Cleanroom Certification
An official certification that the cleanroom satisfies the required cleanliness standards is known as a cleanroom certification. ISO 14644-1, which categorizes cleanrooms according to the number of particles permitted per cubic meter of air, defines these standards. The cleanroom is routinely inspected after certification to guarantee that the set standards are being followed.
Need fast and reliable services for cleanroom validation pharmaceutical services in India? Connect with PDVD Consultancy for ISO 14644-1 cleanroom validation.
Call (+91) 84691 49494 for More Information.

Our Cleanroom Validation Services
1. Facility Design and Layout
2. Installed Filter System Leakage Test
This test makes sure your cleanroom’s ULPA or HEPA filters are operating correctly. We use Poly Alpha Olefin (PAO) integrity testing to check for leaks and make sure the filters meet regulatory requirements.
3. Test for Non-Visible Particle Counting
A thorough investigation of the cleanroom’s airborne particle levels is provided by our particle count test. In accordance with ISO 14644-1 and EU GMP requirements, the test is carried out in three different settings (As-Built, At-Rest, or Operational) to make sure the environment satisfies the necessary cleanliness level.
4. Recuperation Exam
The recovery test calculates the speed at which a contaminated cleanroom can be cleaned up and restored to its initial state. This test is especially significant for sectors like pharmaceuticals, where ensuring rapid recovery times is essential.
5. Airflow Pattern Test (Using Water Fogger)
The Airflow Pattern Test uses water foggers to show the cleanroom’s airflow. Experts make sure that the cleanroom air is flowed evenly and that there are no spots around by using this test. We ensure that Air flow pattern in Clean room is Laminar and not turbulent, Also air flow is as per design pressure zoning between adjacent rooms from higher pressure to lower pressure zone (Room).
6. Balance of Air Pressure
To avoid contamination, the proper pressure differential between cleanroom zones must be maintained. To ensure that the cleanroom keeps the necessary pressure levels, our professionals perform room pressurization tests.
7. The Test of Containment
By using the containment test, you can make sure that particles in the air from nearby locations don’t get inside the cleanroom. We can assist you in keeping the cleanroom under control by finding possible leaks in the building’s structure.
Why Choose us for Pharmaceutical Cleanroom Validation Services in India?
We, at PDVD Consultancy, are proud to provide thorough cleanroom validation services that are customized to your particular requirements. Our skilled group of experts is qualified to carry out all required examinations and assessments with accuracy and consideration. To maintain the efficiency and compliance of your cleanroom, we employ state-of-the-art machinery and follow industry guidelines.
To make sure your facility complies with legal requirements and keeps a production environment under control, the cleanroom validation process is crucial. Certainly, PDVD Consultancy can assist you if you require continuous testing and monitoring or certification for a new cleanroom. You may be confident that your cleanroom will run as efficiently as possible with our professional services, safeguarding both your personnel and your products.
Following are some of the reasons of our success:
- Strong focus on quality compliance.
- Timely delivery of physical service and support.
- Provide effective document as per international guideline.
- Physical presence and urgent document support provide during critical audit.
- Service with answers “today” is our credo to support you with a fast and reliable service by specially trained service engineers to serve your specific needs.
Top Benefits
- Onsite Facility for validation / Qualification / Temperature Mapping Services.
- Qualified and Well-Trained Engineer / Technician Team
- Quality assurance department for exhaustively reviewed All reporting and documentation work thoroughly reviewed as per regulatory guideline
- We have Separate Quality Assurance and Documentation Department to accomplish clients all complicated activity on urgent basis during Audit.
- All Reporting work complies National / International and Major regulatory body like USFDA, MHRA, MCC, TGA, WHO, FDA, ISO etc
- Our motto is punctual Services and Fast Reporting work with in very sort time.
- On-Site Calibration & Validation Facility.
- Remind Customers for due date of Equipment Validation activity by Mail/Telephonic/SMS.
Our Validation Services

Temperature
Mapping

Equipment Validation (Thermal Mapping)

Cleanroom Validation/ HVAC / HEPA Mounted Equipment Qualification

Other Services
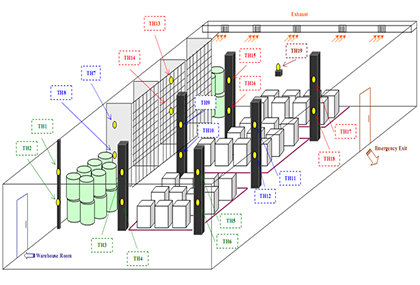
Temperature mapping
Walk in stability chamber Humidity chamber Cold chamber Cold room Deep freezer Refrigerator Incubator BOD incubator Heating block HOT air oven / Vacuum oven Storage area (retain sample room, control sample room, RM store FG store, quarantine) Our Ambition Is Our Main Motto To perform the cleanroom validations, Equipment qualification and thermal mapping activity of area and equipment traceable to international standards and make feel customer pleasant with regulatory audits like as WHO, USFDA, TGA, MHRA, EU, PICs, MCC, Schedule M Etc. by convention the requirements of mandatory tests, test methods and documentation as per the regulatory guidelines and the above regulatory bodies.
We Can Help You Get The Certificate Required for Your Company