Introduction
At PDVD Consultancy, we are experts in providing ready-to-use turnkey pharma solutions for the pharmaceutical and medical industry. We focus on planning, designing, validation, and documentation to ensure your projects are executed excellently from launch to finish.
PDVD Turnkey Pharma Solutions
Our services cover every critical aspect of your project:
- Facility Planning: Customized solutions to optimize layout, workflow, and regulatory compliance.
- Design Consulting: Innovative and cooperative designs that are adaptable to future needs.
- Validation Services: exact validation processes to ensure all systems meet industry standards.
- Documentation Services: Complete documentation to support administrative submissions and audits.

Facility Planning
We develop customized plans that maximize space utilization, streamline processes, and ensure compliance with regulatory requirements, including specialized API planning.

Design Consulting
Our design consulting services Classify efficiency, safety, and future-readiness, working closely with clients to bring their vision to life.

Validation and Documentation Services
We offer thorough validation services for equipment and processes, along with detailed documentation to ensure regulatory compliance and make smooth audits.

Regulatory Compliance and Certification
Navigating the complex landscape of regulatory compliance is our forte. We assist with getting critical certifications like ISO 9001:2015 and compliance with ICH guidelines.


FDA Licensing and GMP Consulting
We provide expert guidance through the FDA licensing process and ensure Good Manufacturing Practice (GMP) compliance, from initial application to final approval.


Cleanroom and HVAC System Validation
Our validation services ensure cleanrooms and HVAC systems meet the highest regulatory standards, reducing polluting risks.
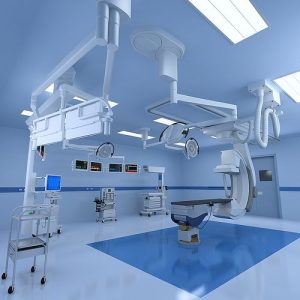
Equipment Qualification and Process Validation
We ensure all equipment and processes are validated to produce consistent, high-quality products, critical for maintaining compliance and quality.
Quality System Documentation
Comprehensive quality system documentation, including Site Master Files and Standard Operating Procedures (SOPs), supports your quality assurance efforts.
Training and Development
We offer specialized training programs on industrial safety and pharmaceutical quality systems, ensuring your team is well-equipped to maintain compliance and operational excellence.
Conclusion
PDVD Consultancy is dedicated to providing top-notch turnkey solutions in planning, designing, validation, and documentation for the pharmaceutical and medical industries. Contact us to find the best turnkey solutions for your projects from expert consultants.
