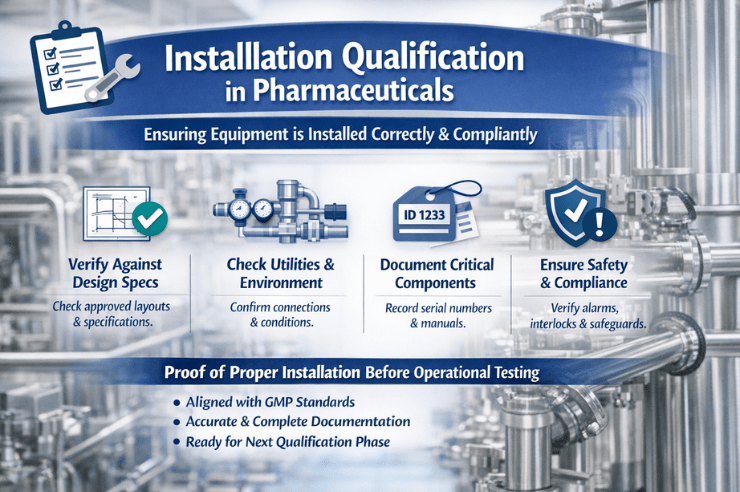
In pharmaceutical manufacturing, equipment earns credibility the hard way. Novelty and sophistication alone do not grant permission to participate in production. Before a single control is engaged or a parameter is tested, the installation itself must withstand scrutiny. This is precisely where Installation Qualification (IQ) takes its place—quietly decisive, methodical, and unforgiving of shortcuts.
IQ is not a clerical ritual performed to satisfy an auditor’s checklist. It is the first real demonstration that a system has been positioned, connected, and configured exactly as it was intended to be. For pharmaceutical manufacturers and the facility partners who bring these systems to life, a rigorously executed IQ converts physical setup into defensible compliance.
Installation qualification in pharma is a formally documented verification that equipment, instruments, systems, and utilities have been installed in alignment with approved design specifications, manufacturer guidance, and
GMP expectations.
Stripped to its essence, IQ answers one uncompromising question:
Is this system installed correctly, without assumptions, and ready to move forward?
IQ occupies the space between design qualification (DQ) and operational qualification (OQ). If IQ is weak or incomplete, everything that follows—testing, validation, performance—rests on an uncertain footing.
What is Installation Qualification, and Why Does Installation Qualification Carry Regulatory Weight?
Regulatory authorities do not rely on supplier reputation or engineering intent. They expect evidence. Tangible, traceable, and contemporaneous evidence that the installation was executed as approved.
Installation Qualification matters because it:
Establishes firm control over physical configuration
Confirms adherence to sanctioned drawings and specifications
Verifies the presence and integrity of critical components
Confirms that utilities and environmental prerequisites are satisfied
Mitigates failure during operational and performance testing
From a compliance standpoint, IQ proves that quality was embedded at the moment of installation, not retrofitted after issues emerged.
Systems and Equipment Subject to Installation Qualification
Manufacturing and packaging equipment
HVAC systems and cleanroom assemblies
Water systems, including PW and WFI loops
Utilities such as compressed air, steam, and process gases
Laboratory and analytical instruments
Environmental and monitoring systems
Any asset capable of influencing product quality, data reliability, or patient safety warrants installation qualification.
Core Elements of a Pharmaceutical IQ Protocol
Verification Against Approved Design
IQ confirms that installation matches approved layouts, engineering drawings, and user requirement specifications (URS). Dimensions, materials of construction, component orientation, and configuration are all examined with intent.
Minor discrepancies—an altered pipe run or substituted material—can escalate into GMP concerns if they remain undocumented or unjustified.
Utility Interfaces and Environmental Conditions
Installation Qualification verifies that all required utilities are correctly connected and meet predefined parameters. Electrical supply, water quality, air pressure, temperature, humidity, and drainage are assessed for suitability. With these, PDVD consultants, the
pharmaceutical consultant in India can help significantly.
For facility systems, this step ensures the surrounding infrastructure does not undermine stability or compliance once operations begin.
Equipment Identification and Record Integrity
IQ requires confirmation that equipment is clearly identified and traceable. Serial numbers, model identifiers, and asset tags must align with documentation.
Supporting records—manuals, drawings, certificates, spare parts lists—are reviewed and approved as part of the qualification dossier, not gathered retroactively.
Instrumentation and Calibration Readiness
Any instrument influencing control or measurement is identified during IQ. While full calibration may occur later, IQ ensures instruments are installed correctly, accessible, and appropriate for calibration.
This foresight prevents downstream delays caused by overlooked or improperly installed devices.
Safety and Compliance Verification
Installation Qualification also confirms that safety mechanisms are present and correctly installed. Guards, interlocks, alarms, and emergency stops must align with specifications and intended use.
Within GMP environments, safety and quality are inseparable disciplines—each reinforcing the other.
The Role of Facility Providers During IQ
Facility service providers occupy a central role in installation qualification, particularly for utilities, HVAC systems, and cleanroom environments. Their responsibilities commonly include:
Executing installations in accordance with approved drawings
Supporting inspection and verification activities
Supplying installation records and as-built documentation
Coordinating closely with quality and
validation teams
Ensuring work remains aligned with GMP requirements
When facility teams understand the intent behind IQ, installation proceeds with fewer interruptions, and qualification timelines remain intact.
Common Installation Qualification Pitfalls
Audit findings often trace back to familiar weaknesses:
Incomplete or fragmented installation records
Unapproved deviations from sanctioned layouts
Insufficient verification of utility connections
Field modifications executed without change control
Poor traceability between equipment and documentation
These issues rarely arise from negligence. More often, they stem from blurred accountability or misalignment between engineering, facilities, and quality functions.
Best Practices for a Robust IQ Process
A successful Installation Qualification relies on preparation rather than correction. Proven practices include:
Finalizing and approving designs before installation begins
Engaging quality teams early in the project lifecycle
Maintaining contemporaneous documentation throughout installation
Managing deviations through formal change control
Conducting thorough reviews before IQ approval
When IQ is treated as a core project phase—not a final administrative hurdle—compliance becomes far more predictable.
Long-Term Value of Proper Installation Qualification
Installation flaws often surface later as repeated deviations, unstable performance, or unexpected downtime. A disciplined IQ process reduces these risks by ensuring systems are correctly established from the outset.
Across the equipment lifecycle, IQ supports:
More efficient operational qualification
Greater confidence during performance qualification
Reduced recurrence of deviations
Stronger outcomes during regulatory inspections
The effort invested during IQ continues to pay dividends long after installation is complete.
Installation Qualification as Proof of Control
In pharmaceutical manufacturing, compliance begins well before a system is powered on. Installation Qualification is the first concrete demonstration that quality principles are being applied where it matters—on the ground, in real installations.
For pharmaceutical organizations and their facility partners, a disciplined IQ process establishes assurance. Assurance that systems are installed correctly, risks are managed deliberately, and GMP expectations are met before production ever begins.
Installation Qualification is not merely about equipment. It is about control, accountability, and readiness—long before the first batch is produced.