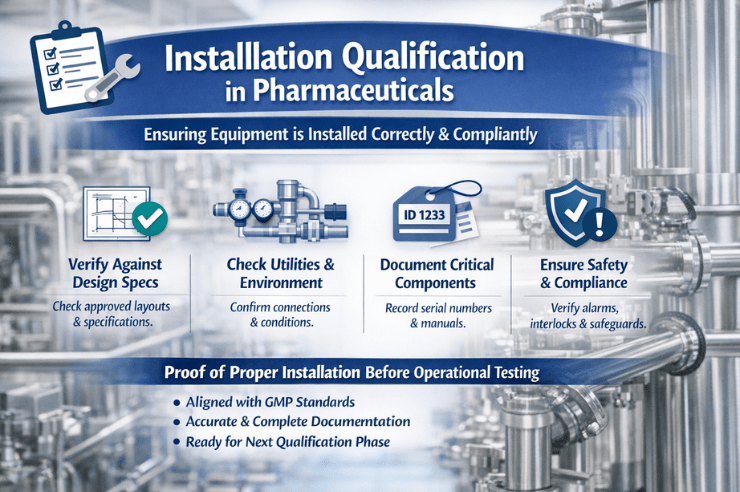
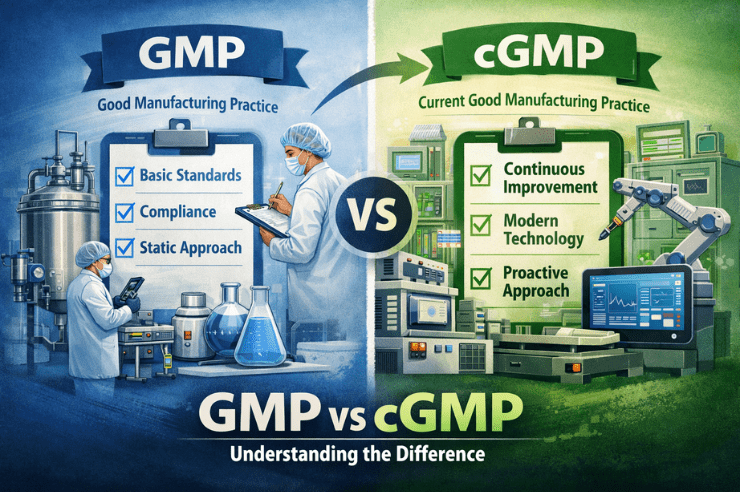
Installation Qualification in Pharmaceuticals
In pharmaceutical manufacturing, equipment earns credibility the hard way. Novelty and sophistication alone do not grant permission to participate in production. Before a single control is engaged or a parameter